From Magic Lamp to Lighting the World: The Pioneering Journey of LEDs

In the early 1960s, the world was on the cusp of a lighting revolution, though few realized it at the time. Nick Holonyak, Jr., a determined engineer at General Electric, sat in his lab, hunched over an experiment that he knew had the potential to change everything.
Holonyak had been working on a phenomenon called electroluminescence—the way certain materials emit light when electric current passes through them. Back then, most lighting relied on incandescent bulbs or fluorescent lights. Incandescent bulbs create light by passing electricity through a filament, heating it until it glows. However, these bulbs are inefficient, with only a small fraction of the energy used being converted into light and most wasted as heat. Fluorescent lights offered a bit more efficiency, but they still used toxic chemicals and had limited lifespans. These traditional lighting options also lacked the versatility and precision of the absolutely phenomenal LEDs, which could be adjusted for color, brightness, and direction.
Light-emitting diodes, or LEDs, are small yet powerful light sources that have revolutionized the way we use lighting in everyday life. Unlike traditional light bulbs, LEDs produce light through the movement of electrons in a semiconductor material. They are highly efficient, long-lasting, and versatile, making them invaluable across a wide range of applications. From digital displays on smartphones and computers to streetlights, vehicle headlights, and even medical equipment, LEDs are everywhere, playing a crucial role in countless devices and infrastructures we rely on daily. Many modern technologies and conveniences we take for granted would not be possible without LEDs. Their compact size, low energy consumption, and durability have made them the go-to choice for both industrial and consumer lighting needs.
At its core, an LED consists of a semiconductor material, usually made from compounds like gallium arsenide or gallium nitride. When a voltage is applied, electrons flow across a junction in the semiconductor from the “n-type” (negative) side to the “p-type” (positive) side, crossing an energy band gap. As they recombine with electron holes on the opposite side by crossing the gap, they release energy in the form of photons, creating visible light. The color of light emitted depends on the band gap of the semiconductor material, which is why different materials produce different LED colors.
Though infrared LEDs, invisible to the human eye, had already been invented in 1961, no one had managed to create a visible LED. Holonyak’s breakthrough came in 1962 when he developed the world’s first visible-spectrum LED, which glowed red. It was dim by today’s standards, but it was revolutionary. Holonyak knew he was onto something significant; he called his LED a “magic lamp,” believing it was the future of lighting.
In the early 1970s, Herbert Maruska and Jacques Pankove at RCA developed the first green LED, marking the next significant step forward. Green LEDs expanded the potential for multicolor applications, particularly in digital displays and signaling, where red and green could be combined to convey more information. However, developing green LEDs posed unique challenges, as it required semiconductor materials that could emit light at a higher energy level (and thus a shorter wavelength) than red LEDs. This required meticulous adjustments to the semiconductor composition, further advancing the field.
Yet, the journey was far from over. Red and green LEDs allowed for rudimentary displays, but without blue, there could be no full-color displays, no way to make true white light. Blue became the elusive target, the missing piece that researchers chased for decades. The main barrier was the band gap requirement—a high-energy gap in the semiconductor’s electronic structure that was tough to achieve without a material that was both stable and manufacturable. This was due to the short wavelength of blue light, which required a large band gap between the semiconductor layers. Countless researchers tried and failed, thwarted by the technical demands of creating a stable blue LED.
Then, in the 1990s, in a quiet corner of Japan, a researcher named Shuji Nakamura took up the challenge. Working for Nichia, a small chemical company, Nakamura poured years into a single goal: creating a blue LED. He used gallium nitride (GaN), a compound that had shown promise in theory but was notoriously difficult to grow. Many believed it was impossible. But Nakamura’s unconventional approach to crystal growth led to a breakthrough in 1994: the first efficient, bright blue LED. Nakamura’s breakthrough made the blue LED a reality, earning him the Nobel Prize in Physics in 2014 alongside Isamu Akasaki and Hiroshi Amano, who also contributed to blue LED research. The creation of the blue LED was revolutionary, as it enabled the RGB (red-green-blue) color model, allowing full-color displays and paving the way for white LEDs, which combine all three primary colors.
Gallium nitride was difficult to work with due to its high melting point and challenging synthesis process. Other researchers had tried and failed to produce stable blue light using similar materials, but Nakamura’s meticulous research into crystal growth and material engineering led to a breakthrough in creating efficient, bright blue LEDs. The development of blue LEDs finally enabled white LED lighting, combining red, green, and blue to produce a spectrum of white light. This was transformative, allowing LEDs to become viable replacements for incandescent and fluorescent lighting.
Each color’s invention had taken the combined knowledge, tenacity, and imagination of multiple scientists, each determined to overcome obstacles that had stymied those before them. The blue LED, especially, was a triumph of resilience, a testament to Nakamura’s refusal to accept the limits imposed by existing materials and methods.
Today, LEDs are everywhere, though we rarely pause to consider the path they took to get here. They’re in the screens we use every day, in streetlights and car headlights, in hospitals, and in our homes. LEDs power medical devices, disinfect water, and even help grow plants indoors. They’ve enabled a world where light is not just illumination but information, connectivity, and even healing. The development of the LED was a journey of discovery that reshaped our relationship with light. It is a story of visionaries who, step by step, brought us a little closer to the light—forever changing the way we see the world.
Similar Post You May Like
-
CFCs, HFCs and their long, troubled history
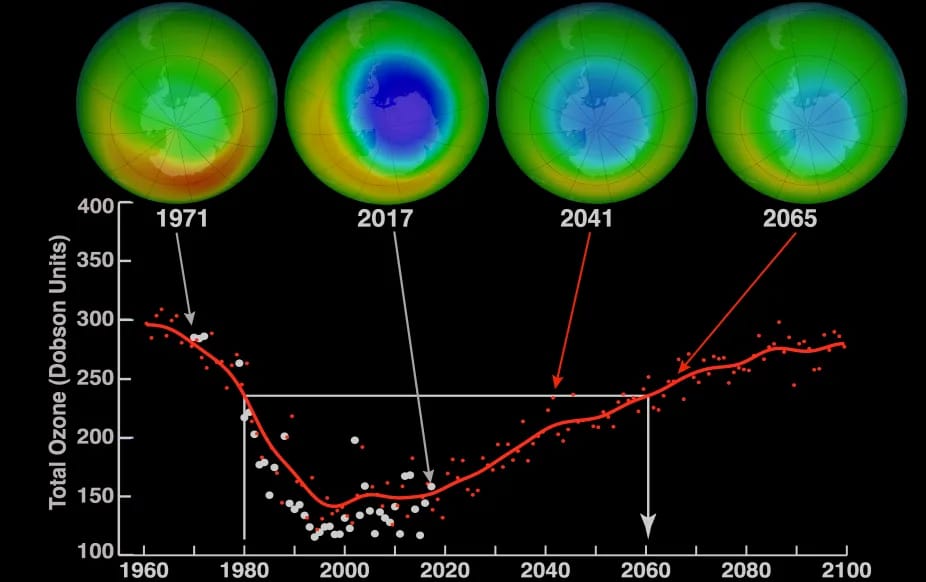
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.






