Pills or shells? DECODING the right choice

It’s common knowledge that most medications in healthcare are tablets and capsules; both are oral dosage forms taken via the mouth as pharmacotherapy, and both show effects after passing through the gastrointestinal tract and the acidic stomach environment to finally reach the site where they are needed. However, there must be a specific reason formulation scientists developed tablets soon after the commercial success of capsules.
The first tablet was made by Grid Systems’ GridPad in the US in 1989, and the first hard capsules were made in 1833 in France by pharmacists Joseph Gérard Auguste Dublanc and François Achille Barnabe Mothes. A tablet is a solid dosage form made from compressed active ingredients combined with excipients such as binders, disintegrants, and coatings to form its shape. Tablets can be produced in various shapes, sizes, and colors, and may include numerical designs to indicate dose adjustment. Capsules have an outer shell, usually gelatin or plant-based, containing the active ingredient in powder or liquid form. They are categorized as hard-shelled (powder or pellets) or soft-shelled (liquid gels). The shell dissolves in the digestive tract, releasing the active ingredient for absorption and circulation.
Tablets are chemically stable, suitable for prolonged storage, can be chewable or split for dose adjustment, and allow larger amounts of active ingredients compared to capsules. They are available in immediate release (IR), delayed release (DR), and extended release (ER) forms. However, tablets can have slower dissolution, bitter aftertaste, and uneven breakdown in the digestive tract, potentially reducing bioavailability.
Capsules dissolve quicker, allowing faster absorption, mask medication flavor, and deliver a greater proportion of active ingredient to the bloodstream. Cons include susceptibility to degradation from humidity and light, limited space for high-dose medications, and gelatin-based shells may not suit all patients.
Healthcare professionals decide between tablets and capsules based on patient-specific therapeutic needs, pathophysiology, and pharmacokinetic considerations. Capsules are ideal for unit doses with no splitting required, while tablets are suitable for dose adjustments for patients with kidney or liver compromise.
In conclusion, tablets provide chemical stability, dose flexibility, and diverse release profiles, making them ideal for chronic therapy. Capsules offer rapid dissolution, better palatability, and improved bioavailability, benefiting fast therapeutic onset and patient compliance. The choice is determined by aligning drug properties with patient physiology and therapeutic requirements.
Author's Note: My name is Duaa Ahmed, a pharmacy undergrad student passionate about consuming and writing content across all media. My goal is to explore and share knowledge widely, and I hope you enjoy my articles as much as I enjoy writing them.
Similar Post You May Like
-
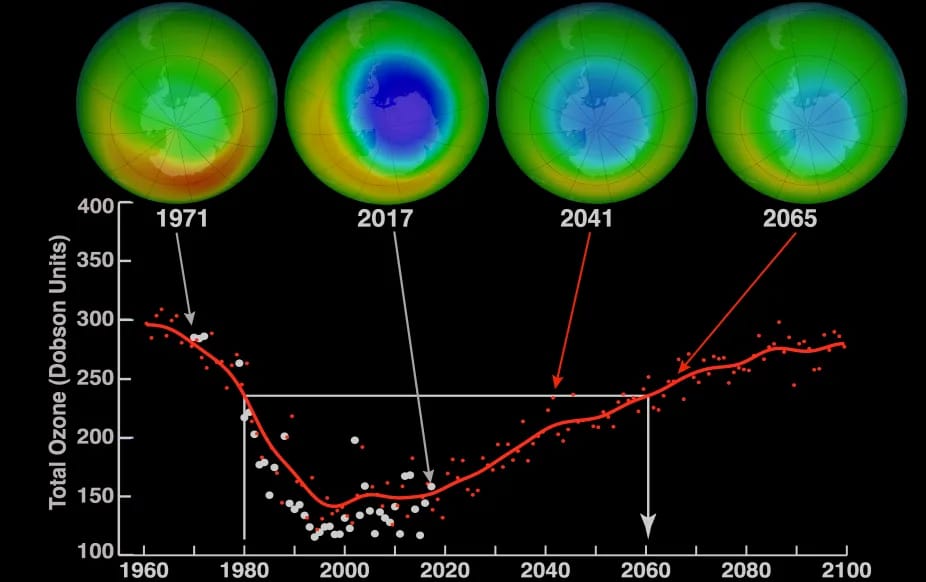
CFCs, HFCs and their long, troubled history
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.






