Genetic Frontiers

“The greatest history of life on Earth is written in DNA.” — James Watson
All living organisms — whether flora, fauna, or human — possess within them a complex and intricate instruction manual. This manual is written in a biological code known as DNA (Deoxyribonucleic Acid), which forms the foundation of genetic code. DNA governs a multitude of characteristics and processes: from an individual’s height and eye color to the regulation of internal biological functions and the ability to resist or combat illness.
For centuries, this minute code lay undecipherable — a mystery in wait. That was until 1953, when scientists James Watson and Francis Crick, used based on out built on the foundational work of Rosalind Franklin and Maurice Wilkins, revealed the double helix structure of DNA. This discovery revolutionized contemporary biology and established the foundations upon which genetic science exists today.
The Science of Reading and Rewriting Life:
The contemporary science of genetics goes far, beyond the task of simply reading the genetic code. Scientists have created sophisticated methods that enable us to edit the code itself. The most revolutionary innovation here is CRISPR-Cas9, a gene-editing system that works like a molecular scalpel. Discovered by researchers Emmanuelle Charpentier and Jennifer Doudna, CRISPR enables scientists to pinpoint regions of DNA and to cut, insert, or manipulate genetic material with unprecedented precision.
To make a comparison, think of the DNA code as a long, and elaborate instruction book. Genetic engineering permits researchers to find mistakes in the book and correct them. In more sophisticated uses, it permits adding altogether new instructions that can provide increased disease resistance, increased agricultural production, or keeps hereditary disorders from being passed along.
Unlocking the Potential — With Responsibility:
The possibilities of such breakthroughs are enormous and potentially revolutionary. Genetic research is already being used to investigate cures for long-standing genetic diseases like sickle cell anemia, muscular dystrophy, and some cancers. It is also being harnessed in agriculture to produce crops more tolerant of pests, disease, and the vagaries of weather — enhancing worldwide food supplies.
But with these scientific breakthroughs also come multifaceted ethical issues.Human genome editing is a profound undertaking that demands caution and responsibility. A minor mistake in genetic alteration can have unforeseen, even harmful, effects. Additionally, the potential of germline editing means there are deep issues of consent, fairness, and possible abuse of technology at stake.
The Legacy of Discovery:
One key early figure in the history of genetics is Barbara McClintock, a Nobel laureate cytogeneticist whose identification of “jumping genes” (transposons) discovered that DNA fragments could migrate within the genome. Her work revolutionized our understanding of the flexibility and adaptability of the genome, supporting the concept that DNA is not a fixed script but a dynamic, responsive system.
McClintock’s research reminds us that the more we know about the genetic code, the more complicated we see it to be — and our role in playing with it.
A Future Written in Code:
As we advance in genetic technology, the international scientific community has to keep the promise of utilizing these technologies responsibly and fairly. Genetic science has the potential to redefine medicine, agriculture, and even human potential. But as is the case with all great tools, they have to be utilized cautiously, wisely, and with integrity.
In the words of James Watson, life’s story is told in DNA. As we look to the future, we are not merely learning to read that story — we are learning to write new chapters.
Beyond the Sequence — The Rise of Epigenetics:
While sequencing and editing DNA provides extraordinary power to understand and alter our genetic blueprint, a rapidly advancing frontier lies in how genes are expressed — not just what they are. This field is known as epigenetics. Rather than altering the genetic code itself, epigenetics involves chemical modifications that switch genes on or off, determining which parts of the DNA are actively read by the cell.
Every cell in the human body carries the same DNA, yet a skin cell behaves, in an entirely differently from a neuron. This is because epigenetic markers — such as methyl groups added to DNA, or modifications to histone proteins around which DNA is wrapped — control gene activity. These modifications act like highlighters or bookmarks in our instruction manual, guiding how the code is interpreted without changing the underlying sequence.
What makes epigenetics especially intriguing is that it is responsive to the environment. Stress, diet, exposure to toxins, physical activity, and even social experiences can influence gene expression through epigenetic mechanisms. These changes can sometimes be passed down to future generations, raising profound questions about inheritance, personal responsibility, and the social determinants of health.
Moreover, epigenetics opens up new avenues for medical research and treatment. Conditions like cancer, Alzheimer’s disease, and autoimmune disorders are increasingly being linked to faulty epigenetic regulation. Therapies targeting epigenetic changes — such as drugs that modify DNA methylation patterns or histone acetylation — are already in development and, in some cases,undergoing clinical use.
In a broader sense, epigenetics reminds us that our biology is not entirely predetermined. While our genes provide the script, how that script is performed — or even whether it’s read at all — can change over time, shaped by the lives we lead. It underscores the delicate interplay between nature and nurture, genetics and environment, and science.
Citations:
Watson, J. D., & Crick, F. H. C. (1953). Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature.
Charpentier, E., & Doudna, J. A. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science.
McClintock, B. (1950). The origin and behavior of mutable loci in maize. Proceedings of the National Academy of Sciences.
National Human Genome Research Institute (nhgri.nih.gov)
World Health Organization: Human Genome Editing: Recommendations (2021)
i
Similar Post You May Like
-
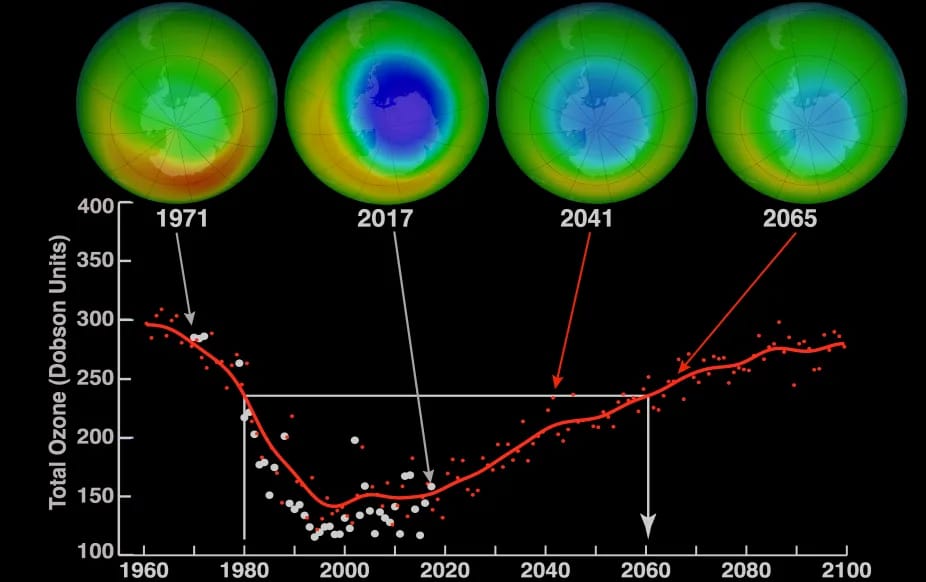
CFCs, HFCs and their long, troubled history
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.






